
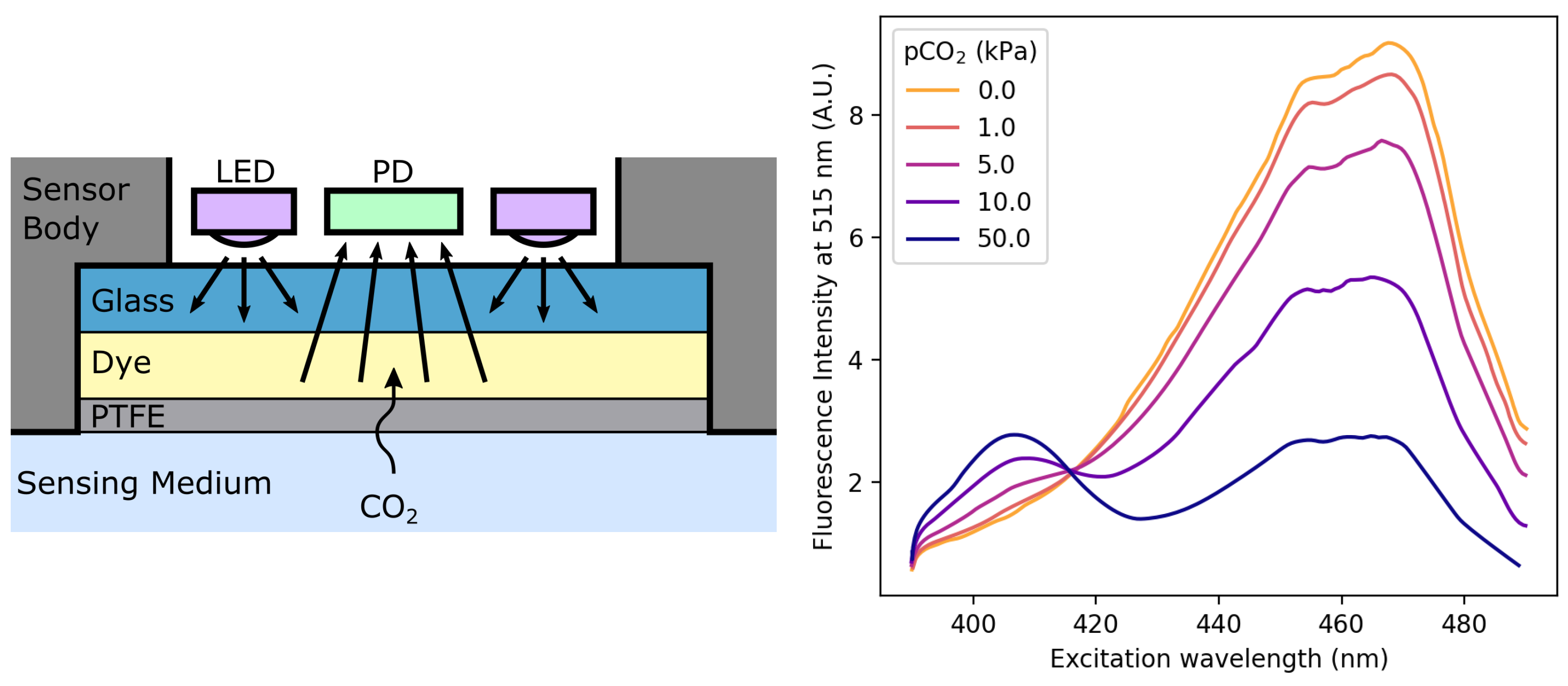
440 MMHG TO ATM HOW TO
If the pressure is increased to standard pressure and the temperature remains constant, what will the new gas volume be P1V1 P2V2. Task: Convert 975 torrs to mmHg (show work) Formula: Torr ÷ 1 mmHg Calculations: 975 Torr ÷ 1 975 mmHg Result: 975 Torr is equal to 975 mmHg Conversion Table For quick reference purposes, below is a conversion table that you can use to convert from Torr to mmHg. Learn how to convert from pascals to atm and what is the conversion factor as well as the conversion formula. A sample of oxygen gas has a volume of 150 mL when its pressure is 440 mmHg. If 1 atm has to be converted to bar, $1atm\,=\,1. 1.0 atm 760 mmHg 101.3 kPa And 0 C 273 K. Convert an altitude in feet or metres of height above sea level to a pressure reading in millibar (mbar, mb or mbr), pounds per square inch ( psi ), millimetres of mercury at zero degrees celsius ( mmHg 0 deg C) or inches of mercury at zero degrees celsius ( inHg 0 deg C). Let’s see some interconversion from 1 atm. Visit 440 Kilopascal to mmHg Conversion Millimeter Mercury (0C) : Millimeter of mercury is a small pressure unit which represents the pressure pushing down due to gravity of any volume of liquid mercury which is 1mm high. 0.58 atm 440.8 mmHg: mmHg: 0.19 atm 144.4 mmHg: 0.39 atm 296.4 mmHg: 0.59 atm 448.4 mmHg: mmHg: 0.2 atm 152 mmHg: 0.4 atm 304. Note: We know the interconversion between the units to do various calculation problems. So we can expresses the equation of pressure as, We know that pressure is the force exerted in a specific area and it is calculated by dividing the force applied by the area on which the force was applied. 0.042585002 atm (standard atmosphere) 0.043999994 at (technical atmosphere) 32.364601844 torr 440 mmH2O (mm of water) 32.364601844 mmHg (mm of mercury) 1.274196606 inHg (inches of mercury) 0.625827676 psi (pounds per sq. The formula to convert from mmHg to atm is: atm mmHg 760 Conversion Example Next, let's look at an example showing the work and calculations that are involved in converting from millimeters of mercury to atmospheres (mmHg to atm). So before going into the solution part of the question, let’s discuss some concepts about pressure and its unit. In the question, it is asked that we have to convert the given value of unit in mm Hg to atmospheres of pressure.The question is simply about te unit conversion of values between the units of pressure,

We should be familiar with 1 atm value equal to how much value of mmHg. Please visit pressure conversion to convert all pressure units.Hint: The approach for the question should be done by focusing on the unit conversion of the value from mmHg to atmospheres of pressure in atm.
Pascal is a metric pressure unit and is equal to one force of newton per square meter. Torr) is a pressure of fluid from one millimeter of mercury. To convert pascal to torr, multiply the pascal value by 0.00750061685 or divide by 133.322368. Boyles Law states that the pressure of a fixed mass of gas at a constant temperature is inversely proportional to its volume. Pascal = torr * 133.322368 How to convert pascal to torr (mmHg)?ġ Pascal (Pa) is equal to 0.00750061685 torr (mmHg). 142.05mL From the information given for this question, we can see that this kind of situation is involving Boyles Law. To convert torr to pascal, multiply the torr value by 133.322368. How to convert torr (mmHg) to pascal?ġ Torr (mmHg) is equal to 133.322368 pascals (Pa). To convert torr (mmHg) to pascal (Pa) or to convert pascal to torr (mmHg), you may use the converter above.īelow, you will find information of how to convert torr to pascal and how to convert pascal to torr, including the formulas and example conversions. A sample Of oxygen gas has a volume Of 150 ml- When its pressure is 440 mmHg.


 0 kommentar(er)
0 kommentar(er)
